An Ultrasound Can Now Treat High Blood Pressure?
A new ultrasound device can help calm overactive nerves and help bring blood pressure down to normal levels.
This article is more than 2 years old
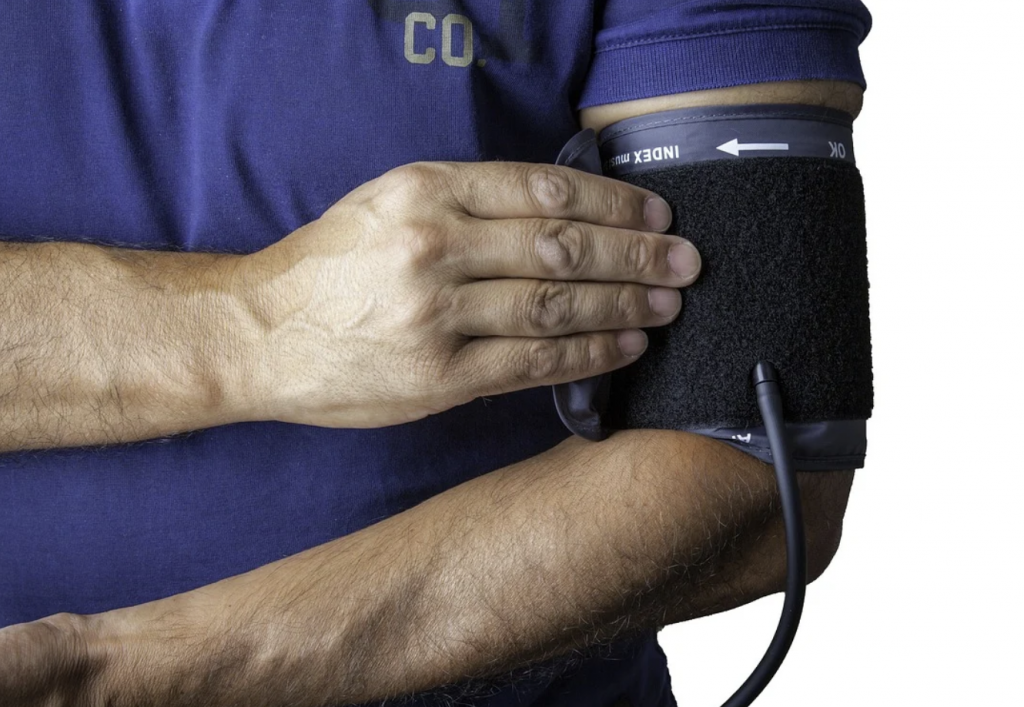
High blood pressure, or hypertension, is a condition where the blood flow pressure through the arteries is continually elevated. Although there are many causes of high blood pressure, the condition is linked to overactive kidney nerves in some middle-aged people. Now, a new ultrasound device can help calm these overactive nerves and help bring blood pressure down to normal levels.
A joint study by researchers at Columbia University and the Université de Paris in France found that the use of the renal ultrasound device resulted in a consistent lowering of daytime ambulatory (at-home) blood pressure. The outpatient procedure, ultrasound renal denervation, led to an average reduction of 8.5 points in middle-aged individuals suffering from hypertension. Because each division on the American Heart Association’s blood pressure chart is separated by 10 points, this is a significant reduction.
Over time, untreated hypertension can lead to myriad health problems, including strokes, heart attacks, heart failure, and irreversible kidney damage. Doctors usually recommend lifestyle changes, such as losing weight and reducing sodium intake, to lower blood pressure. Medications are also available to treat hypertension, but about one-third of people with high blood pressure are unable to control their hypertension with medicine or lifestyle changes.
“Many patients in our clinical practice are just like the patients in our study, with uncontrolled blood pressure in the 150s despite some efforts,” said Ajay Kirtane, MD, co-leader of the study and professor of medicine at Columbia University Vagelos College of Physicians and Surgeons. Kirtane is also an interventional cardiologist and director of cardiac catheterization laboratories at New York-Presbyterian/Columbia University Irving Medical Center. “Renal ultrasound could be offered to patients who are unable to get their blood pressure under control after trying lifestyle changes and drug therapy before these events occur,” he said.
In middle-aged people, hypertension is often caused by overactive renal nerves that cause the body to retain water and sodium. These nerves also release hormones that raise blood pressure. Some antihypertensive medications work by removing excess fluid or lowering the pressure-raising hormones, but none of them directly target the faulty renal nerves.
Ultrasound renal denervation is delivered to overactive nerves inside the kidney’s artery via a thin catheter. The catheter is inserted into a vein in the wrist or leg and threaded into the kidney artery. Ultrasound is then transmitted to calm the overactive nerves that cause high blood pressure, and most people see results within one month of treatment.
The study combined data from three different randomized trials that included more than 500 middle-aged people with varying degrees of hypertension. Study patients also had varying levels of high blood pressure medication use. Twice as many people who received the ultrasound therapy achieved their target daytime blood pressure of less than 135/85 mmHg compared to those who didn’t receive it.
The study’s investigators say the treatment could be used in addition to traditional medications and lifestyle changes in those with uncontrolled hypertension. “Once the device is available, we envision recommending it to patients who have tried other therapies first. The hope is that by controlling blood pressure, we might be able to prevent kidney damage and other effects of uncontrolled blood pressure,” Kirtane said. Kirtane noted that the procedure was well-tolerated by most patients and the majority of them were discharged by the hospital on the same day. Although the results of the study published in JAMA Cardiology are promising, the use of the ultrasound device is still investigational. Ultrasound renal denervation has not been approved by the FDA yet for use outside of clinical trials but will be evaluated later this year.